Course 4
A path to model based biomanufacturing
6 Modules | 4 hours
This online course, “A Path to Model Based Biomanufacturing,” comprises six modules, covering the basics of models and modeling, digital twins for biomanufacturing, modeling of pharmaceutical processes, modeling packages and platforms, a modeling lifecycle framework, and investment considerations for model-based biomanufacturing. The course provides a big-picture path for managers and executives through modules 430, 431, and 440, and enables learners to understand models and modeling, digital twins, and investment considerations, along with practical applications of modeling in pharmaceutical processes.
This course offers
- Access from any device
- Certificate of completion
- 4 hours online course content
This module aims to introduce you to models and modeling. You will cover questions such as:
- What is a model?
- What is the power of models?
- What pitfalls should you avoid when using models?
- What is the vision of a model-based enterprise?
The learning objectives of this module are that you will be able to:
- Describe some of the drivers for modeling
- Define key performance indicators that can be improved using models
- Explain some of the key pitfalls to avoid when using models
- Describe the vision of a model-based enterprise
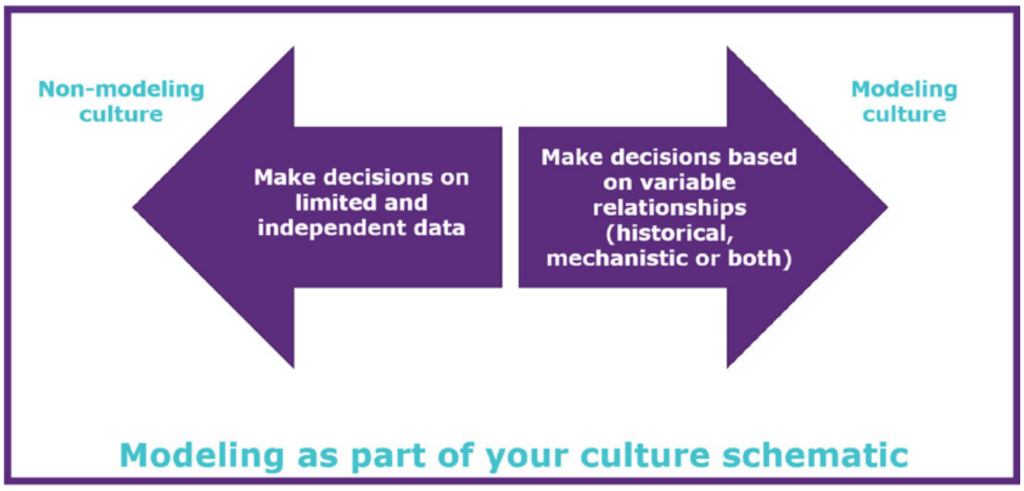
This module aims to introduce you to the concept of digital twins and the different types of digital twins used for biomanufacturing. You will cover questions such as:
- What are the benefits of model-based biomanufacturing?
- What are the 4Ps of modeling for Life Sciences manufacturing?
- What is a digital twin?
- What is the difference between a digital model and a digital twin?
The learning objectives of this module are that you will be able to:
- Explain key examples of the benefits of model-based biomanufacturing
- Describe the 4Ps of biopharmaceutical manufacturing
- Describe what a digital twin is
- Explain the key differences between a digital model and a digital twin
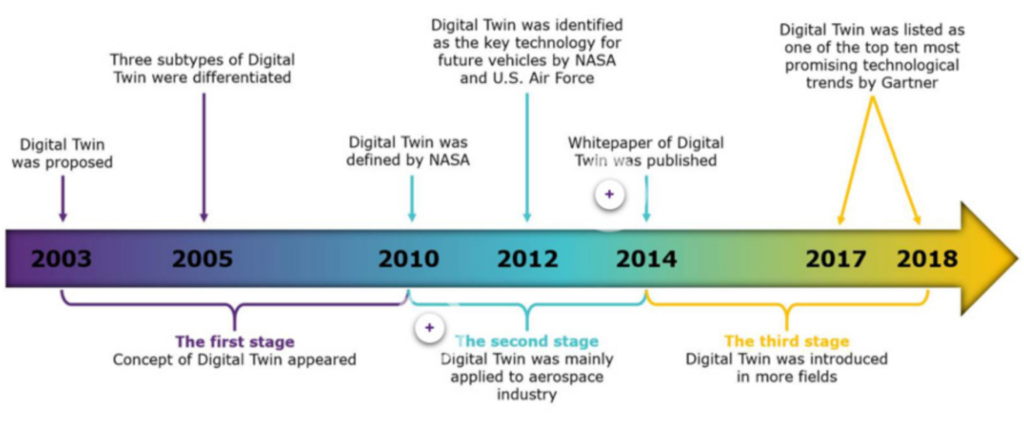
This module aims to introduce you in more detail to modeling of pharmaceutical processes. You will cover questions such as:
- How can modeling and simulation support Quality by Design workflows?
- How does Process Analytical Technology (PAT) rely on modeling?
- What are the most common objectives of modeling?
- What are some of the different applications of modeling for biomanufacturing?
- What are the three main types of modeling approaches?
- What do you need to be aware of if your objective changes?
- Which models are most suited to batch or continuous processes?
- What are typical examples of models used in the biopharmaceutical industry?
The learning objectives of this module are that you will be able to:
- Describe different types of models that can be used for different objectives and applications
- Explain how Process Analytical Technology relies on modeling
- List which type of modeling and which models are suited to various stages of product development of manufacturing (e.g. batch or continuous processes)
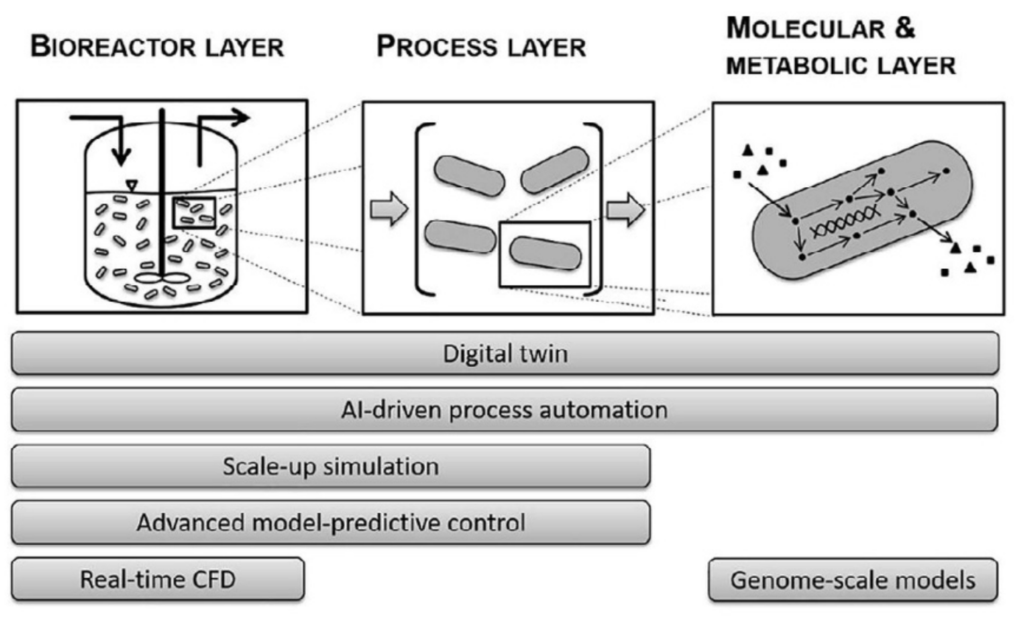
- What are some of the main statistical and modelling packages used to perform data analysis or create models?
- List a number of packages that are used for modeling
- Define the manufacturing stage and context models are most suitable for
This module aims to introduce you to managing models using a lifecycle framework. You will cover questions such as:
- Why is model life-cycle management so important?
- How are models assessed differently in an industrial context?
- What are the main steps in a model lifecycle framework?
The learning objectives of this module are that you will be able to:
- Explain why lifecycle management of models is important
- Describe the main steps in a model lifecycle framework
- Describe a modeling portfolio management process
This module aims to introduce you the factors that impact investment in model-based technologies. You will cover questions such as:
- What are the business drivers and economics of model based biomanufacturing?
- What are the technology investment considerations?
- What are the personnel investment considerations?
- What is the future of model-based biomanufacturing?
The learning objectives of this module are that you will be able to:
- State key business drivers for model-based biomanufacturing
- Outline factors relating to technology investment
- Outline personnel investment considerations
- Describe the impact on tech transfers of products
from one site to another - Explain some of the key equations that support data-driven economic decisions

